The Voice of China on the Global Stage: Darwin Biotech Shines at the 2025 World Orphan Drug Congress Europe

From October 27 to 29, 2025, the World Orphan Drug Congress Europe 2025—a leading conference in the field of orphan drugs and rare diseases—was held at the RAI Convention Centre in Amsterdam, the Netherlands. The event brought together more than 2,000 industry representatives, 250+ distinguished speakers, and over 130 exhibiting companies. The agenda covered a full spectrum of topics, from clinical development, gene and cell therapies, and protein therapeutics to policy, regulation, and market access.
As an innovative biotechnology company in China’s cell-based biopharmaceutical sector, Darwin Biotech was invited to attend and delivered an oral presentation highlighting its latest research achievements in neural repair and stem cell–derived therapeutic proteins.
A Global Platform for Rare Diseases
The World Orphan Drug Congress, organized by Terrapinn and co-hosted by EURORDIS (Rare Diseases Europe) and IRDiRC (International Rare Diseases Research Consortium), is one of the most influential professional conferences in the field of rare diseases and orphan drugs in Europe and worldwide.
The 2025 conference, themed “Covering the Full Lifecycle of Orphan Drugs,” featured multiple thematic tracks focused on key areas such as clinical development, precision medicine, regulatory policies, pricing and reimbursement, real-world evidence, and patient engagement. Participants included pharmaceutical companies, research institutions, regulatory agencies (such as the EMA), patient organizations, and investment firms.
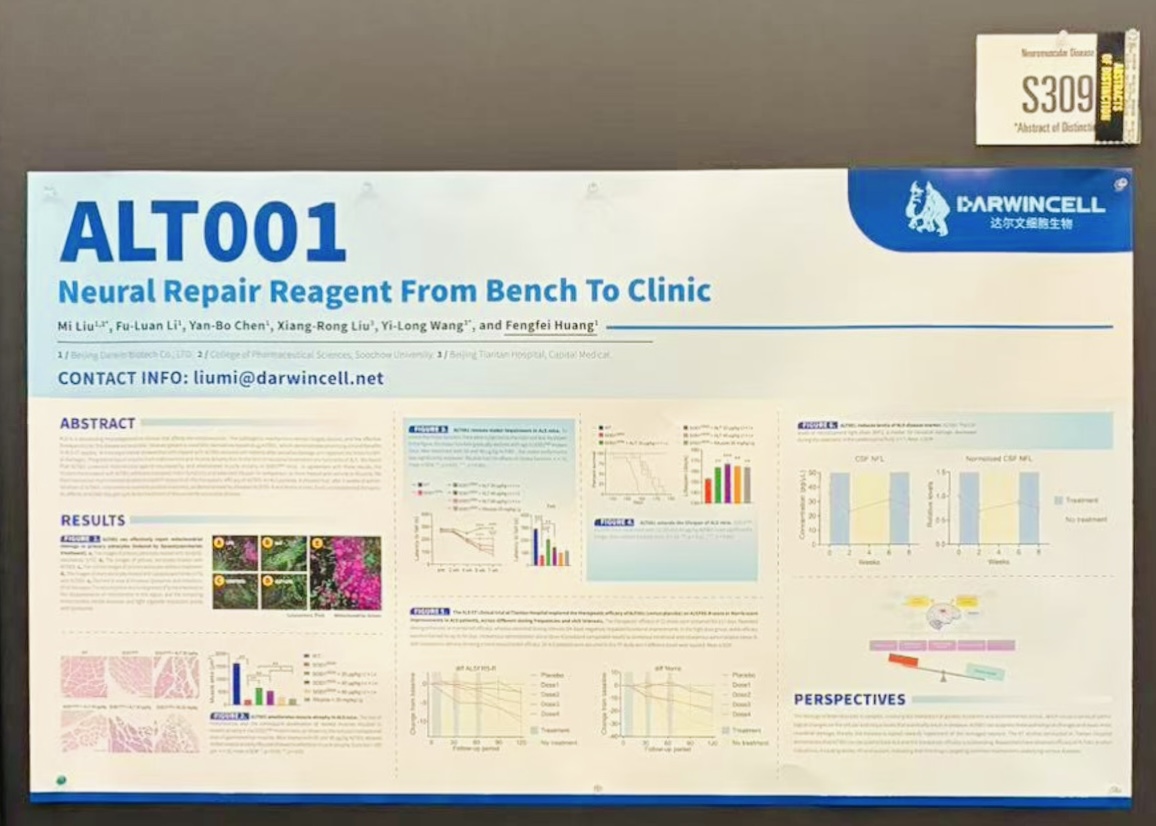
In the “Biopharma Showcase” session, Professor Liu Mi, Chief Scientific Officer of Darwin Biotech, delivered a talk titled “From Bench to Clinic:ALT001, Therapeutic Protein Complex Derived from Stimulated Stem Cells, Repairs Neuronal Functions in ALS Patients” The presentation provided a comprehensive overview of the development concept, mechanism of action, and preclinical findings of ALT001.

Targeting ALS with Neural Repair Therapies
Amyotrophic lateral sclerosis (ALS), one of the world’s most well-known “orphan diseases,” remains a major unmet medical challenge due to its small patient population and high treatment complexity. Characterized by progressive skeletal muscle weakness and atrophy, ALS often leads to respiratory and circulatory failure in the late stage and shows an increasing trend of younger onset.
In China, the incidence and prevalence of ALS are approximately 0.6 per 100,000 and 3.1 per 100,000, respectively, with an average survival period of 3–5 years.
Currently, global treatment options—limited to riluzole, edaravone, and tofersen—only delay disease progression without maintaining or reversing neurological function. Thus, there remains an urgent global demand for innovative ALS therapies.
To address this unmet need, Darwin Biotech is advancing the development of ALT001, a first-in-class neurorepair protein therapeutic. Derived from stress-induced mesenchymal stem cells using the company’s proprietary Stem Cell Stress Induction Platform, ALT001 exerts multi-mechanistic effects, including reducing oxidative damage, modulating mitochondrial and lysosomal function, suppressing neuroinflammation, and promoting neural repair—offering a new therapeutic pathway for ALS patients.
Significant Preclinical and Clinical Progress
ALT001 has achieved multiple research milestones:
• In vitro studies demonstrated its ability to restore the viability of oxidatively damaged cells.
• In the SOD1G93A mouse model, ALT001 protected motor neurons, mitigated muscle atrophy, and improved both motor function and survival compared with the placebo and riluzole groups.
• In investigator-initiated clinical trials involving dozens of ALS patients, ALT001 showed encouraging outcomes.
A randomized, double-blind clinical study conducted at Beijing Tiantan Hospital further confirmed that ALT001 treatment improved both ALSFRS-R scores (ALS Functional Rating Scale–Revised) and NfL (neurofilament light chain) levels, laying a crucial foundation for advancing its global orphan drug development program.
Expanding Global Collaboration
During the congress, Darwin Biotech engaged in in-depth discussions with multiple European research institutions, pharmaceutical companies, and patient organizations to explore collaborative opportunities in clinical research, regulatory submissions, and market access.
This participation further expanded the company’s global academic and industrial network, setting the stage for its future international strategic initiatives.
Continuous Innovation for Global Patients
Looking ahead, Darwin Biotech will continue to focus on innovative protein polymer therapeutics, driven by unmet clinical needs. The company aims to accelerate the clinical translation of ALT001 and related candidates, strengthen global partnerships, and bring new treatment options to patients suffering from rare neurological diseases.
About Orphan Drugs
Orphan drugs refer to therapies developed for the prevention, diagnosis, or treatment of rare diseases. Due to the limited patient population and high R&D costs, the field has long struggled with a lack of commercial incentives.
To encourage innovation, regions such as the United States, European Union, and Japan have enacted Orphan Drug Acts that provide benefits including accelerated registration, market exclusivity, and tax incentives.
While more than 600 orphan drugs have been approved worldwide, over 7,000 rare diseases still lack effective therapies.
The continued innovation and global collaboration in orphan drug development remain a key driving force for the pharmaceutical industry.
Darwin Biotech remains committed to deepening international cooperation with research institutions, clinical centers, pharmaceutical companies, and patient organizations—advancing the clinical and commercial progress of ALT001 and other innovative therapeutics—to bring hope to rare disease patients around the world.
About Darwin Biotech
Beijing Darwin Cell Biotechnology Co., Ltd., founded in 2016, is a national high-tech enterprise dedicated to innovative therapies for neurological diseases.
The company has established a comprehensive technology platform spanning from protein discovery to clinical translation, based on its proprietary ECIWEP® (Extracted Cell-Interacting Water-Soluble Protein) technology.
Through close collaborations with leading medical and research institutions, Darwin Biotech strives to become a global leader in neurorepair biotechnology innovation.
Popular Post
Categories
- Research Insights (02)
- Laboratory Best Practices (04)
- Innovation & Technology (01)
- Industry Trends (03)
- Sustainability in Science (05)
- Events & Workshops (02)
- Educational Resources (04)